The Biolochanics project
Biolochanics is the name of a 5 years project which started in May 2015 and which has the ambitious objective of preventing aortic aneurysm from being a cause of mortality. Remember that the rupture of Aortic Aneurysms (AA) kills more than 30000 persons every year in Europe and the USA.
The project is led by Prof Stéphane Avril and gathers together a team of about 20 researchers involving permanent faculty, postdocs and PhD students.
It has the financial support of the European Research Council (ERC) which attributed a grant of 2 million euros to Prof Stéphane Avril in 2015 (ERC-2014-CoG BIOLOCHANICS).
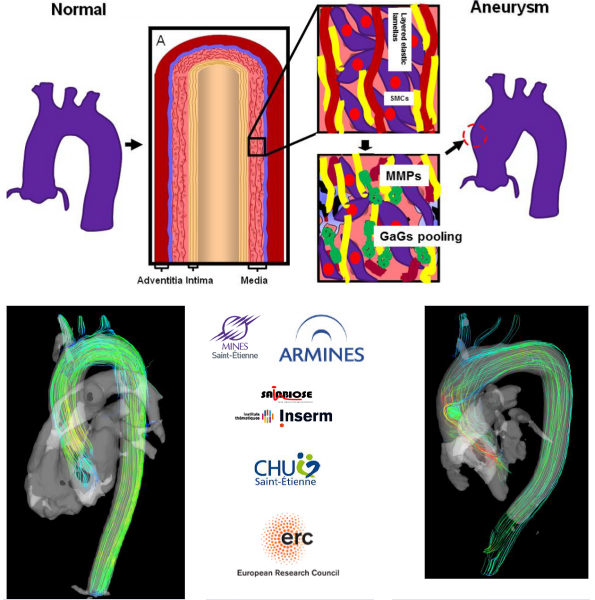
The Biolochanics vision
The rupture of an aortic aneurysm is a complex phenomenon that occurs when the wall stress exceeds the local strength of the aorta due to degraded properties of the tissue. Our vision is that the evolution of the strength and of the wall stress of the aorta during the growth of an aneurysm can be predicted on a patient-specific basis by a computational model. We plan to develop this computational model and to validate it on different cohorts during the course of the Biolochanics project. The use of the computational model will be proposed to cardiac and vascular surgeons for a prediction of the mid-term and long-term fate of every aortic aneurysm. This will permit to plan surgical intervention more securely and more timely for the benefit of all the patients.
The Biolochanics roadmap
An aortic aneurysm may be considered as a mechanobiological instability. While the tissue is healthy, uniform stress and stiffness properties are maintained across the aorta according to the principle of homeostasis. The tissue is mechanobiologically stable. But when the mechanosensitivity of cells in the tissue is impaired or when the regulation of structural proteins is chemically disturbed, a mechanical heterogeneity may develop irreversibly in the tissue and may initiate an aneurysm.
Our aim is to develop finite-element models of aortas that intrinsically account for the regional variations of material properties in the tissue. These regional variations of material properties will be determined for every patient at the moment of their examination. They will constitute the initial conditions of a model that will predict their evolution and its consequences on the integrity of the tissue.
The important stages of the project are the following:
- Development of the computational model. The local effects of proteolytic remodeling in the aortic tissue are first investigated in vitro using full-field optical measurements and inverse identification of the distribution of material properties. Then, the changes in the regional distribution of material properties occurring at different stages of aneurysm progression are identified combining biaxial testing, full-field optical measurements and inverse identification on a model of mice aneurysm. Finally, a new constitutive model able to account for local tissue remodeling is implemented in a finite-element environment. It takes into account internal length-scales controlling localization mechanisms involved in the development of the mechanobiological instability. This resorts to the nonlocal continuum damage theory.
- Patient-specific calibration of the computational model. Based on a new approach combining 4D phase-contrast magnetic resonance imaging (PC-MRI), fluid-structure interaction (FSI) finite element simulations and inverse identification, we perform a complete patient-specific reconstruction of the distribution of material properties in the wall of the aorta and of the blood action applied on it. All the patients diagnosed with an aneurysm of the ascending thoracic aorta at the Saint-Étienne University hospital and in other hospitals willing to collaborate are invited to take a 4D MRI exam. The distribution of their aortic material properties is reconstructed from these MRI data using the new approach. Two groups of patients are considered: i. patients having a surgical intervention after the exam: their excised tissue is collected and tested mechanically in order to determine if the material properties determined from the MRI are correct; ii. patients remaining under surveillance: they are invited to take the exam six months later in order to characterize the kinetics of growth and remodeling. The data are used to calibrate the model predictions.
- Clinical validation. An user-friendly interface will be developed and tested by a group of clinicians. For patients willing to take a PC-MRI exam, it will permit: 1. reconstructing automatically the model of each patient’s aneurysm, 2. visualizing the existing heterogeneities of material properties at the date of the exam, 3. predicting the growth of the aneurysm and the remodeling of the material properties over the next 12 months.
The Biolochanics team
People from the CIS center for biomedical and healthcare engineering
| Stéphane AVRIL | Responsable du département STBIO | |
| Miquel AGUIRRE | Maitre Assistant Associé | |
| Nicolas CURT | Technicien de laboratoire | |
| Solmaz FARZANEH | Post doctorante | |
| Jérôme MOLIMARD | Enseignant-Chercheur | |
| Claire MORIN | Enseignant-chercheur | |
| Baptiste PIERRAT | Ingénieur de recherche | |
People from the CHU-SE university hospital
- Salvatore Campisi is cardiac surgeon at CHU-SE. He runs the clinical cohort of patients for the Biolochanics project
- Bertrand Chavent is vascular surgeon at CHU-SE. He works on histological analyses of aneurysms
Former members of the group
- Francesca Condemi was a post-doc fellow from 1st April 2016 to 31st March 2018. She worked on CFD analyses to derive hemodynamic descriptors in ATAA patients. She is now with the University of Toronto.
https://www.researchgate.net/profile/Francesca_Condemi
- Frances Davis was a post-doc fellow from 1st September 2013 to 31st January 2015. She worked on biomechanical characterization of aneurysmal tissue. She is now with the University of Southampton.
https://www.researchgate.net/profile/Frances_Davis2
- Olfa Trabelsi was a post-doc fellow from 1st July 2016 to 31st August 2018. She worked on the biomechanical effects of proteolytic remodeling in the aortic tissue. She is now with the University of Technology of Compiègne (France) at https://bmbi.utc.fr.
- Victor Acosta wwas a post-doc fellow from 1st July 2016 to 31st August 2018. He worked on the measurement of strain fields in aortic tissues using optical coherence tomography (OCT) and digital volume correlation (DVC).
- Edouard Breysse was a master student in 2017-2018, coming from University of Saint-Étienne. She worked on the biomechanical effects of proteolytic remodeling in the aortic tissue. .
- Rossella Campobasso was a master student in 2017-2018, coming from Politecnico Torino. She worked on FSI analyses to derive hemodynamic descriptors in ATAA patients. .
- Ambroise Duprey was a vascular surgeon at CHU-SE and phD student from 1st October 2013 to 31st May 2015. He worked on biomechanical characterization of aneurysmal tissue. He is now Professor at the University of Reims (France) and head of the vascular surgery department at the Reims University Hospital.
Visitors
 Nele Famaey is a research associate at KUL, Belgium. She visits our group for 3 months in 2016 and works on computational modeling of aneurysm growth.
Nele Famaey is a research associate at KUL, Belgium. She visits our group for 3 months in 2016 and works on computational modeling of aneurysm growth.
https://www.mech.kuleuven.be/en/bme/research/soft-tissue-biomechanics
 Mirunalini Thirugnanasambandam was a PhD student at the University of Texas San Antonio, USA, under the supervision of Prof Ender Finol. She works on the Clinical Management of Abdominal Aortic Aneurysms (AAA) using Patient-Specific Tissue Mechanics. She visited our group from September to December 2015. We are developing together a method of stress analysis for aortic aneurysms which does not require running a finite element model.
Mirunalini Thirugnanasambandam was a PhD student at the University of Texas San Antonio, USA, under the supervision of Prof Ender Finol. She works on the Clinical Management of Abdominal Aortic Aneurysms (AAA) using Patient-Specific Tissue Mechanics. She visited our group from September to December 2015. We are developing together a method of stress analysis for aortic aneurysms which does not require running a finite element model.
 Matthew Bersi was a PhD student at Yale, USA, under the supervision of Prof Jay Humphrey. He works on the mechanical properties of the aorta of different mouse models of aneurysms. We developed together a method able to derive regional variations of these material properties. He visited our group in December 2015.
Matthew Bersi was a PhD student at Yale, USA, under the supervision of Prof Jay Humphrey. He works on the mechanical properties of the aorta of different mouse models of aneurysms. We developed together a method able to derive regional variations of these material properties. He visited our group in December 2015.
- Jay D Humphrey is John C. Malone Professor and Chair of Biomedical Engineering at Yale, USA. He has 30 years of experience in the field of continuum biomechanics, with primary interest in vascular mechanics and mechanobiology. Stéphane Avril visited his group from May to August 2014 and continues visiting regularly. The collaboration is on an inverse method able to derive regional variations of mechanical properties in the aorta of different mouse models of aneurysms. Jay visited our group in June 2017 and received the Honoris Causa doctorate from IMT.
- Yiqian He from Dalian University (China) obtained a funding from the National Scientific Foundation of China (NSFC) which has an agreement with the ERC. Under the framework of this agreement, Prof Yiqian He was hosted in our group from February 2018 to July 2018. He works on a specific theoretical point posed by the implementation of the growth and remodelling model in the BIOLOCHANICS project: how to assign nonlocal constitutive properties in the model, which would permit more realistic models of aneurysm growth.
- Di Zuo was master student from Dalian University (China) supervised by Prof Yiqian. He was hosted in our group from March 2018 to July 2018.
- Anne M Robertson is Professor in the Department of Mechanical Engineering and Materials Science, Swanson School of Engineering, University of Pittsburgh. She visited us in February/March 2018. She holds several grants about biomechanics of cerebral aneurysms.
Close collaborations
 Magalie Viallon is a physicist and engineer at CHUSE. She is a specialist of MRI. With Prof Pierre Croisille, head of radiology at CHUSE, they play a key role in acquiring the best image data possible on the recruited patients.
Magalie Viallon is a physicist and engineer at CHUSE. She is a specialist of MRI. With Prof Pierre Croisille, head of radiology at CHUSE, they play a key role in acquiring the best image data possible on the recruited patients.
https://www.creatis.insa-lyon.fr/MUST/FR/equipe/
 Jia Lu is Professor at the University of Iowa, USA. He is an expert in inverse problems for soft membranes. The collaboration is on the local identification of mechanical and rupture properties in aneurysm samples tested with a bulge inflation system.
Jia Lu is Professor at the University of Iowa, USA. He is an expert in inverse problems for soft membranes. The collaboration is on the local identification of mechanical and rupture properties in aneurysm samples tested with a bulge inflation system.
 ANSYS contributes to the Biolochanics project by supplying support and licenses for their finite element package.
ANSYS contributes to the Biolochanics project by supplying support and licenses for their finite element package.
- Marco Evangelos Biancolini is Professor at Dipartimento di Ingegneria dell’Impresa “Mario Lucertini” at Università di Roma Tor Vergata (Italy). He is an expert in mesh morphing. The collaboration is on tracking the deformation of the aortic wall during cardiac cycles.
- Umberto Morbiducci is Professor at Polytechnic University of Torino (Italy). He is an expert in computer fluid dynamics. With him and his collaborator Diego Gallo, we investigate novel hemodynamics descriptors that may be relevant for aneurysmal risk of rupture.
 Nele Famaey is a research associate at KUL, Belgium. She visits our group for 3 months in 2016 and works on computational modeling of aneurysm growth.
Nele Famaey is a research associate at KUL, Belgium. She visits our group for 3 months in 2016 and works on computational modeling of aneurysm growth. Mirunalini Thirugnanasambandam was a PhD student at the University of Texas San Antonio, USA, under the supervision of Prof Ender Finol. She works on the Clinical Management of Abdominal Aortic Aneurysms (AAA) using Patient-Specific Tissue Mechanics. She visited our group from September to December 2015. We are developing together a method of stress analysis for aortic aneurysms which does not require running a finite element model.
Mirunalini Thirugnanasambandam was a PhD student at the University of Texas San Antonio, USA, under the supervision of Prof Ender Finol. She works on the Clinical Management of Abdominal Aortic Aneurysms (AAA) using Patient-Specific Tissue Mechanics. She visited our group from September to December 2015. We are developing together a method of stress analysis for aortic aneurysms which does not require running a finite element model. Matthew Bersi was a PhD student at Yale, USA, under the supervision of Prof Jay Humphrey. He works on the mechanical properties of the aorta of different mouse models of aneurysms. We developed together a method able to derive regional variations of these material properties. He visited our group in December 2015.
Matthew Bersi was a PhD student at Yale, USA, under the supervision of Prof Jay Humphrey. He works on the mechanical properties of the aorta of different mouse models of aneurysms. We developed together a method able to derive regional variations of these material properties. He visited our group in December 2015. Magalie Viallon is a physicist and engineer at CHUSE. She is a specialist of MRI. With Prof Pierre Croisille, head of radiology at CHUSE, they play a key role in acquiring the best image data possible on the recruited patients.
Magalie Viallon is a physicist and engineer at CHUSE. She is a specialist of MRI. With Prof Pierre Croisille, head of radiology at CHUSE, they play a key role in acquiring the best image data possible on the recruited patients. Jia Lu is Professor at the University of Iowa, USA. He is an expert in inverse problems for soft membranes. The collaboration is on the local identification of mechanical and rupture properties in aneurysm samples tested with a bulge inflation system.
Jia Lu is Professor at the University of Iowa, USA. He is an expert in inverse problems for soft membranes. The collaboration is on the local identification of mechanical and rupture properties in aneurysm samples tested with a bulge inflation system.