The evaluation of aerosol therapy medical devices is at the heart of public health studies conducted for companies and communities.
It is based on an approach through experimentation and uses :
- aerosol therapy devices such as inhalers or nebulizers and their improvement (prototyping, tests, feedback).
- vaping devices for adaptation as medication devices (characterization of emissions, toxicology)
- Harmfulness and identification of addictive behavior in a public health approach.
It relies on the Aerosol (aerosol metrology, anatomical models for the study of aerosol deposition) and Toxicity (physico-chemical particle characterization techniques, toxicology tests, exposure at the air/liquid interface) expertise of the Biological Activity of Inhaled Particles department (BioPi)
Examples of projects:
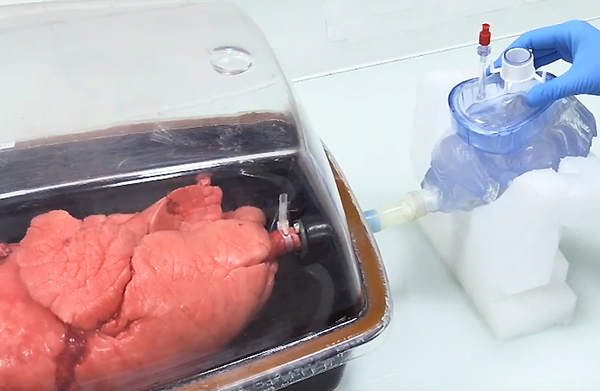
• Study of aerosol deposition in the respiratory tract with a unique model that consists of coupling a 3D printed human head made of plastic to ex vivo pig lungs. The objective is to reduce animal experimentation.
• Conditions of use of the electronic cigarette (comparison with tobacco). Bench testing and determination of “Vape Profiles
• Study of the performance of masks for medical and non sanitary use (bacterial filtration, viral filtration, breathability, decontamination …).
Contact : Lara Leclerc, CIS, dpt BioPi / SAINBIOSE, DVH Team

This technological demonstrator is based on the Inhaled particles technical platform (equipment, expertise and skills) of the Centre for Biomedical and Healthcare Engineering